


|
About Products Blog Gallery Contact |

|
Home > Blog |
Perfecting your pHWhy and how to control the pH of your feed solutionpH (potential Hydrogen) is a measure of the acidity or alkalinity of a substance. The range goes from 0 to 14 with lower values being more acidic, higher values more alkaline and 7 being neutral. The pH of a hydroponic feed solution influences the availability of nutrients, so it needs to be maintained within an optimum range. Plants generally absorb nutrients most effectively when the feed is between pH 5.6 and 6.4. When pH moves outside of this range macro nutrients can become less available, absorption of micro nutrients can reach toxic levels and precipitation (nutrients becoming solids and falling out of solution) can occur in the feed. For example, plants can develop magnesium and calcium deficiency or copper and iron toxicity when pH drops below 5.0, while a pH above 6.5 can cause iron deficiency. 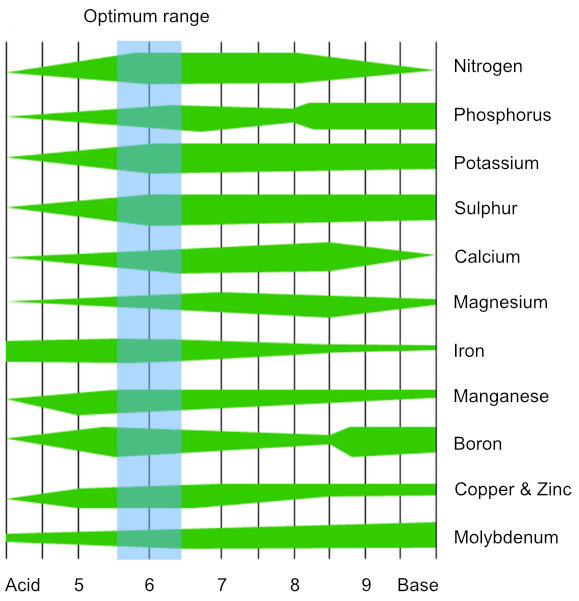
Availability of nutrients at different pH levels How to test pH It is good practice to check the pH of your feed solution every day, at least until you get a feel for how your system behaves. Testing should be done after adding nutrients because nutrients change the pH of the feed. A test can be performed using either pH liquid test drops or a pH meter. Test drops change colour and need to be compared against a colour chart, whereas a meter shows pH as a numeric value on a display. Inexpensive 'pen' style meters, suitable for amateur use, are readily available online and are generally more accurate than liquid test drops. Highly accurate and more expensive meters, available from hydroponic retailers, are the preferred choice of professional growers. Meters require regular calibration (i.e. adjusting to match a calibration solution of known pH; also available online) to ensure that readings remain correct. 
pH measuring equipment How to adjust pH An acid is used to lower pH and a base to increase it. Many acids and bases are extremely corrosive and require careful handling. Since source water is typically above pH 6.4 and pH tends to drift up as plants use nutrients from the feed solution, it is more likely that pH will need to be reduced rather than increased. Several acids are suitable for lowering pH, with nitric acid and phosphoric acid being the most commonly deployed. Mild organic acids such as household vinegar can also be used, however their effect is not long lasting. Hydroponic retailers sell phosphoric acid marketed as ‘pH Down’ and potassium hydroxide/potassium carbonate marketed as ‘pH Up’. It takes only a small amount of either product to change the pH of the feed, so they should be used very sparingly. 
Hydroponic pH Up and pH Down How to manage pH Without soil, plants do not benefit from the many natural interactions that regulate pH. It is therefore helpful to use a hydroponic nutrient with the capacity to buffer the feed solution, such as Hydrocrop HydroSol. Buffering helps to improve pH stability and prevent wild swings. The pH of the feed solution in a recirculating system can be monitored in the supply reservoir. In a media-based system, because pH changes as the feed travels through the growing media and plant root zone, it is advisable to check the pH of the leachate (the solution that drains from the grow bed) also. Make adjustments as necessary to keep pH within the optimum range but be aware that a) some movement within the range is desirable as it improves nutrient availability, and b) adjustments of more than 0.5 per day should be avoided as drastic changes can shock plants. 
Testing feed solution pH Why pH changes Beyond the normal influence of growing plants, there are several reasons why feed solution pH may change over time. High mineral levels in hard water can cause the pH to rise, as can inorganic growing media in media-based systems. Organic matter in the feed solution can also cause wide pH fluctuations, with algae and bacteria being the main culprits. If pH increases in the morning and drops later in the day, algae may be to blame (because algae consume acidic carbon dioxide during the daytime). On the other hand, bacteria from root disease can cause a dramatic drop in pH levels (because bacteria will release acids into the feed solution as diseased roots decompose). Temperature also has an effect because a warm feed will slowly release carbon dioxide into the atmosphere, causing pH to rise. To ensure effective nutrition for your hydroponic plants, it’s important to control the EC of the feed solution as well as the pH. You can read more about EC management and control in our blog article Nice an EC does it. |
|
Website and content © Hydrocrop 2021 |